Abstract
Background:
High expression of the ecotropic viral integration site-1 (EVI-1) is an independent negative prognostic indicator of survival in leukaemia patients. Zebrafish (Danio rerio) is a vertebrate animal model commonly used to examine haematopoiesis and myeloid malignancies. To clarify the molecular mechanisms of EVI-1, we previously introduced the human EVI-1 gene into embryonic zebrafish through a heat-shock promoter and established the stable germ-line Tg(EVI-1: HSE: EGFP) zebrafish (Shen et al, 2013). Arsenic trioxide (As2O3, ATO) is one of the effective anticancer drugs, especially for patients with leukaemia (Udupa et al, 2017). We thus aimed to explore the anticancer effects of ATO and the underlying functions associated with EVI-1 in an in vivo zebrafish model and in AML cells in vitro.
Results:
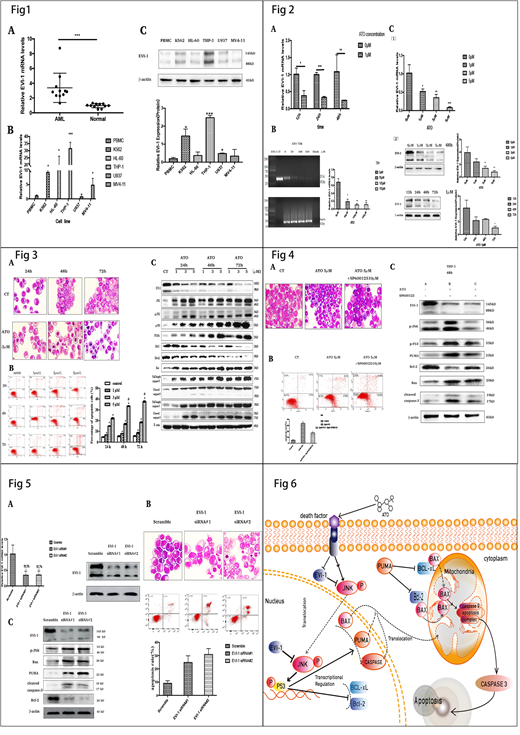
We determined EVI-1 expression in mononuclear cells isolated from the bone marrow and peripheral blood of AML patients and healthy donors by RT-qPCR and Western blot analysis. EVI-1 was highly expressed in primary AML (Fig 1A). Then, EVI-1 expression was detected in five leukaemia cell lines (K562, HL-60, U937, THP-1 and MV4-11) and normal PBMCs. Among these five leukaemia cell lines, THP-1 has the highest EVI-1 expression (Fig 1B and Fig1C).
Primary acute monocytic leukaemia cells from one patient with high expression of EVI-1 were treated with ATO. We found ATO could significantly decrease EVI-1 mRNA (Fig 2A). Between the ATO-treated groups and the control group, the expression of EVI-1 were significantly reduced in the THP-1 cell line (Fig 2B). Next, we evaluated the EVI-1 expression in Tg(EVI-1: HSE; EGFP) transgenic zebrafish embryos over dose courses of ATO exposure (Fig 2C). Consistent with the results of our in vitro study, ATO decreased EVI-1 expression in a dose-dependent manner after 72 h (Fig 2C). Taken together, these results indicate that ATO is an inhibitor of EVI-1 expression both in vivo and in vitro.
We investigate whether the reduction of THP-1 cells viability is due to apoptosis, THP-1 cells were incubated with 3 µM of ATO for 24 h, 48 h or 72 h. In the light microscopy images, THP-1 cells exhibited typical apoptotic characteristics (Fig 3A). The proportion of apoptotic cells was represented as early apoptotic cells (annexin V+/PI- staining, the lower right quadrant) plus late apoptotic cells (annexin V+/PI+ staining, the upper right quadrant) (Fig 3B). In cytometric analysis, ATO increased the percentage of apoptotic THP-1 cells in a dose- and time-dependent manner. We found that ATO increased the expressions of JNK, p-JNK, p-P53, PUMA, Bax, caspase-9 and caspase-3 (including cleaved caspase-9 and -3) but decreased the expressions of Bcl-2 and Bcl-xl (Fig 3C).
To further verify the role of the JNK pathway in ATO-mediated THP-1 cell apoptosis, we examined if the inhibitor of JNK (SP600125) could reverse ATO-induced apoptosis in THP-1 cells. We found SP600125 not only decreased the pro-apoptotic effect of ATO in the THP-1 cell line (Fig 4A and Fig 4B) but also decreased the activation of the JNK-mediated apoptotic signalling pathway (Fig 4C). SP600125 silenced the activation of JNK by completely inhibiting the phosphorylation of JNK but had little effect on EVI-1 expression (Fig 4C).
To test whether EVI-1 modulates apoptosis via the JNK signalling pathway, we transiently transfected THP-1 cells with EVI-1 siRNA which significantly reduced EVI-1 expression (Fig 5A). Silencing EVI-1 had a significant effect on the activation of the JNK pathway and the induction of THP-1 cell apoptosis (Fig 5B and Fig 5C).
Conclusion:
Our study demonstrated that the apoptotic pathway in THP-1 cells induced by ATO is closely associated with the oncogene EVI-1, the pro-apoptotic protein JNK, p-JNK, p-P53, PUMA, Bax, caspase-9 and caspase-3 (including cleaved caspase-9 and cleaved caspase-3), and the anti-apoptotic proteins Bcl-2 and Bcl-xL. ATO can downregulate EVI-1 mRNA and oncoprotein and block the repression of EVI-1 in the JNK pathway. Furthermore, the activated JNK signalling pathway regulated the expression level of apoptosis-associated proteins, including p-P53, PUMA, Bax, Bcl‐xL, Bcl‐2, Bax, caspase-9 and caspase-3(Fig 6). These findings may provide a novel theoretical basis for the development of personalized medical strategies for the treatment of EVI-1 positive AML patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal